

Diamond Unity®
Integrated Electrolyte System
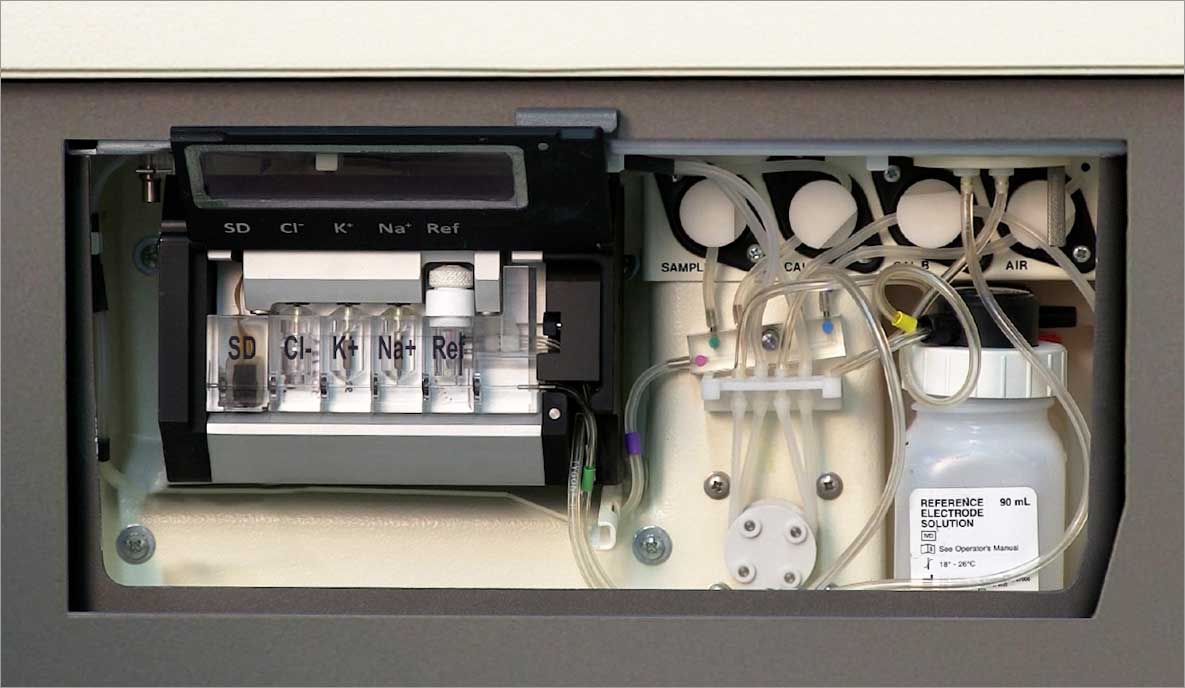
The Diamond Diagnostics Unity Integrated Electrolyte System is a fast and compact ISE module measuring Na+, K+ and Cl- within Serum, Plasma and Diluted Urine utilizing Ion Selective Electrode (ISE) technology. The Diamond Unity® is uniquely designed with a powerful microprocessor and integrated electronics allowing for easy integration into any size Chemistry Analyzer platform.
Featured Benefits
- Compact Size
- Flexible Reagent System
- Long Life Reference
- Accessible Service Area
- Fully Visible Pathway
- Fully Shielded Measurement Chamber
- Positive Engagement Latching System
- Horizontal Electrode Orientation
Built-In Modular Sample Cup Option • Accessible Service Area
| Sample | Number of Parameters | Communication | ||
| Serum or Diluted Urine | 3 (Simultaneously) | RS232 Communication | ||
| Data Storage | Calibration | Analysis Time | ||
| 2000 Patient Results | 2 Point every 8 hours | Serum: 30 sec (100 per hour) | ||
| 500 QC Results per level (3) | On Demand | Urine: 60 sec (50 per hour) | ||
| 100 Calibrations | 1 Point after every Measuremen | |||
| Sample Size | ||||
| 60 - 100 µl Serum | ||||
| 140 - 150 µl Urine | ||||
Simple Integration | ||||
 |  |  | ||
| Custom Parameter Options | Recirculating Reference Electrode | Rapid Integration | ||
Parameters and Specifications | ||||
| Parameters | Range | Reproductibility | Resolution | |||
|---|---|---|---|---|---|---|
| Serum Na+ | 50-200 mmol/L | CV ≤ 1% (120-160 mol/L) | 0.1 mmol/L | |||
| Serum K+ | 1.5-10.00 mmol/L | CV ≤ 2% (2.8-6 mmol/L) | 0.01 mmol/L | |||
| Serum CL- | 50-200 mmol/L | CV ≤ 2% (80-120 mmol/L) | 0.1 mmol/L | |||
| Urine Na+ | 20-500 mmol/L | CV ≤ 5% (60-280 mmol/L) | 1.0 mmol/L | |||
| Urine K+ | 5-300 mmol/L* | CV ≤ 5% (30-170 mmol/L) | 1.0 mmol/L | |||
| Urine CL- | 10-500 mmol/L | CV ≤ 5% (80-230 mmol/L) | 1.0 mmol/L | |||
| *(60-120) requires additional dilution | ||||||
| Power | Size & Weight | Ambient Conditions |
| 12VDC, 2.0A | 92 mm (H) x 134 mm (W) x 46 mm (D) | Room temperature: |
| (self adjusting) | or 335 x 315 x 295 mm | 15-32°C/60-90°F |
| 3.70” (H) x 5.27” (W) x 1.81” (D) | Humidity <85% | |
Literature and Video
The names and logos of manufacturers, their instruments, and their products referred to herein may be protected by trademark or other law, and are used herein solely for purpose of reference. Products are available for international distribution only unless otherwise indicated. Diamond Diagnostics expressly disclaims any affiliation with products it does not manufacture, as well as sponsorship by other manufacturers. For current regulatory status on products within this website, please contact your sales representative. Prices and Information shown are for reference only and may change without notice. SmartLyte®, ProLyte®, CareLyte®, and Down-To-Frame® Refurbishing are Registered Trademarks of Diamond Diagnostics®. No mobile information will be shared with third parties/affiliates for marketing/promotional purposes. All the above categories exclude text messaging originator opt-in data and consent; this information will not be shared with any third parties.

