

Diamond CareLyte® Plus
The Choice for Veterinary Laboratories
The Diamond Diagnostics CareLyte® Plus Electrolyte Analyzer for is a completely automated system measuring Na+, K+, Cl-, Ca++ and Li+ in whole blood, serum, plasma and urine utilizing Ion Selective Electrode (ISE) technology. The Diamond CareLyte® Plus is uniquely designed with a powerful microprocessor allowing for easy interface with external LIS systems, operation with a new touch screen interface, bar code scanner and storage and retrieval of over 10,000 patient results. The system currently operates in over 12 languages and offers testing on Feline, Canine, Bovine, Equine, Swine and Ovine samples. The perfect analyzer for any size Veterinary Laboratory.
Featured Benefits
- 28 Sec Analysis
- 512 Tests/Hour
- 40,000 Results Stored
- Touch Screen Display
- Remote User Access
- Online Service Interface
Fastest Results • Large Storage Capacity • Advanced Functionality
| User Input | Data Storage | Connectivity | ||
| 5” Touch Screen | 10,000 Patient Results | LAN, Wifi, 4 USB ports | ||
| Remote Access | 10,000 QC Results per Level | RS232, LIS | ||
| Analysis Time | Support | Number of Parameters | ||
| 28 sec (512/hour) | Online Service Interface | 5 Total 4 (Simultaneously) | ||
| Calibration | Sample Size | Veterinary Options | ||
| 2 Point every 4 hours | Whole Blood, Serum (95 µl) | Feline, Canine, Bovine, | ||
| 1 Point every sample | Plasma, QCs (95 µl) | Equine, Swine, Ovine, | ||
| On Demand | Urine (180 µl) | Murine or Open | ||
| Languages | ||||
| English, 中文, Español, | 日本語, 한국어, Português, | |||
| Français, Deutsch, | Polskie, Русский, Türkçe | |||
| Indonesia, Italian, 日本語, | ||||
User Friendly Navigation | ||||
 |  | 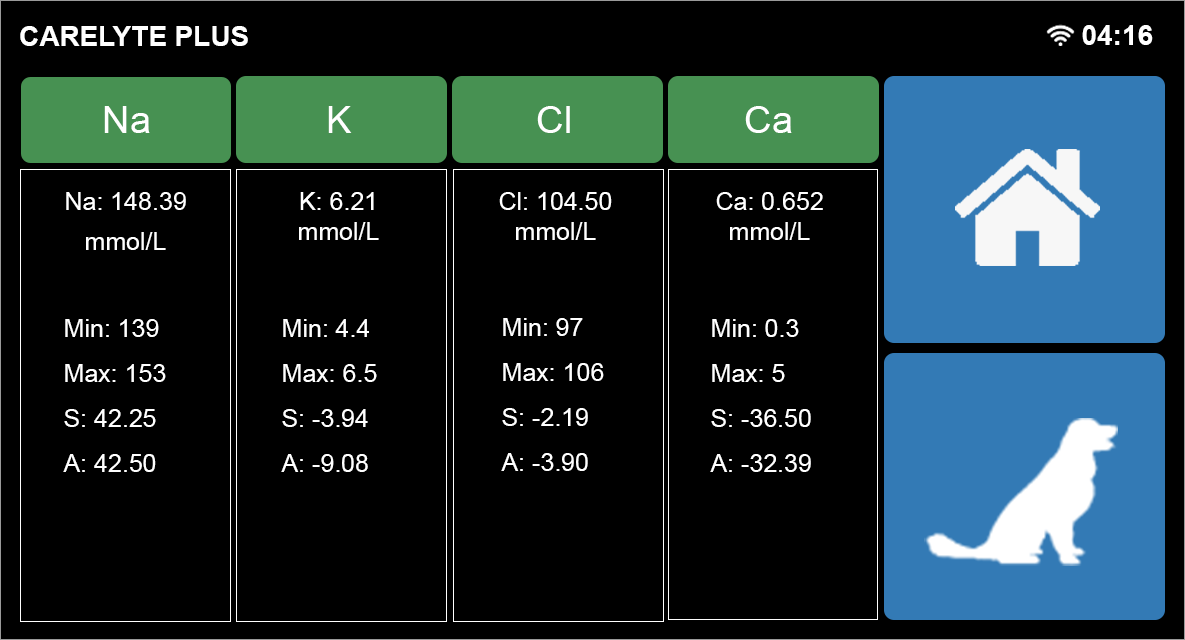 | ||
| User Friendly Navigation | Simple Search Function | Easily Access Data and Results | ||
Parameters and Specifications | ||||
| Parameters | Range | Reproductibility* | Resolution | |||
|---|---|---|---|---|---|---|
| Serum Na+ | 40-200 mmol/L | CV ≤ 1% (120-160 mol/L) | 0.1 mmol/L | |||
| Serum K+ | 1.7-15 mmol/L | CV ≤ 1.5% (2.5-6 mmol/L) | 0.01 mmol/L | |||
| Serum CL- | 50-200 mmol/L | CV ≤ 1% (85-130 mmol/L) | 0.1 mmol/L | |||
| Serum Ca++ | 0.3-5.0 mmol/L | SD ≤ 0.02 mmol/L (0.8-1.5 mmol/L) | 0.001 mmol/L | |||
| Serum Li+ | 0.2-5.5 mmol/L | SD ≤ 0.03 mmol/L (0.4-1.3 mmol/L) | 0.001 mmol/L | |||
| Urine** Na+ | 3-300 mmol/L | CV ≤ 5% (100-250 mmol/L) | 0.1 mmol/L | |||
| Urine** K+ | 5-120 mmol/L*** | CV ≤ 5% (10-60 mmol/L) | 0.01 mmol/L | |||
| Urine** CL- | 15-300 mmol/L | CV ≤ 5% (100-250 mmol/L) | 0.1 mmol/L | |||
| *Typical Within Run (n=30) Blood, Serum, Plasma | ||||||
| **Calcium and Lithium are not typically measured in urine samples | ||||||
| ***(60-120) requires additional dilution | ||||||
| Power | Size & Weight | Ambient Conditions |
| 12VDC, 2.0A | 92 mm (H) x 134 mm (W) x 46 mm (D) | Room temperature: |
| (self adjusting) | or 335 x 315 x 295 mm | 15-32°C/60-90°F |
| 3.70” (H) x 5.27” (W) x 1.81” (D) | Humidity <85% | |
Literature and Video

The names and logos of manufacturers, their instruments, and their products referred to herein may be protected by trademark or other law, and are used herein solely for purpose of reference. Products are available for international distribution only unless otherwise indicated. Diamond Diagnostics expressly disclaims any affiliation with products it does not manufacture, as well as sponsorship by other manufacturers. For current regulatory status on products within this website, please contact your sales representative. Prices and Information shown are for reference only and may change without notice. SmartLyte®, ProLyte®, CareLyte®, and Down-To-Frame® Refurbishing are Registered Trademarks of Diamond Diagnostics®. No mobile information will be shared with third parties/affiliates for marketing/promotional purposes. All the above categories exclude text messaging originator opt-in data and consent; this information will not be shared with any third parties.
